Shows how to draw Lewis Dot Structures for ionic compounds. You can see a listing of all my videos at my website, http://www.stepbystepscience.com.
- Lewis Dot Structure For Ionic Compounds Calculator Answer
- Lewis Dot Ionic Bonding Worksheet
- Ionic Bonding Lewis Dot Diagram
In covalent bonding atoms share electrons.
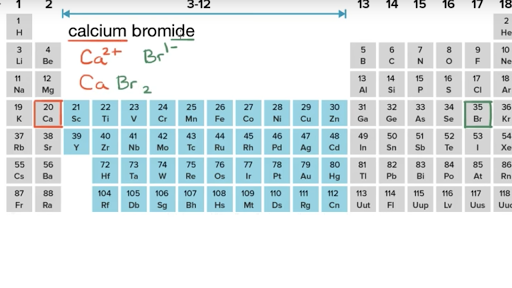
- Figure 7.10 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Figure 7.10 Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons.
- Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. How the molecule might react with other molecules. The physical properties of the molecule (like boiling point, surface tension, etc.).
Take for example the H2 molecule. Each hydrogen atom says, 'I only need one more electron to be like a noble gas (helium) .' Since each hydrogen has only one electron, when two hydrogens get together they can share their electrons.
So each hydrogen atom now sees 2 electrons when it is covalently bonded to another hydrogen atom. Pure hydrogen exists as H2 molecules. The same is true for all of the halogens in column 7A:
- Pure chlorine exists as Cl2
- Pure bromine exists as Br2
- Pure iodine exists as I2
Chemists often use the symbol '-' to represent a bond. For example, H-H is a 'hydrogen molecule' and Cl-Cl is a 'chlorine molecule.' The line in between the two atoms means that they are sharing two electrons between them. Let's take oxygen as another example. Oxygen atoms like to combine to form O2. In this case, each oxygen atom wants 2 more electrons, so when the two oxygen atoms get together they share a total of 4 electrons. We write O2 as:

Chemists call this a double bond. By forming a double bond between them, each oxygen atom can then see as many electrons as a Ne atom has.
Now let's look at nitrogen. It also likes to combine to form a diatomic molecule, in this case N2. Each nitrogen atom, however, wants 3 electrons, so two nitrogen atoms share a total of 6 electrons.
Lewis Dot Structure For Ionic Compounds Calculator Answer
We call this a triple bond.
Of course, you can form molecules from more than one type of atom. Let's look at water. H2O consists of two hydrogen atoms sharing their electrons with one oxygen atom.
Another example is hydrogen peroxide, H2O2.
Think about hydrogen peroxide and decide on your own if all of the atoms are happy with the number of electons around them.
Here is one final example. Carbon atoms want to share 4 electrons, so it is very happy if it can get together with 4 hydrogens to form methane, CH4.
In this example, carbon is sharing 4 electrons with 4 hydrogens and each hydrogen is sharing one electron with carbon.
Structural and Empirical Formulas
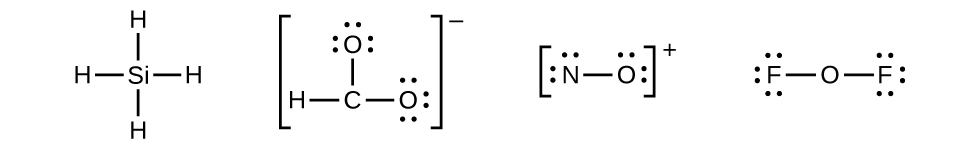
Structural Formula

To avoid confusion, chemists often write the structural formula when identifying a molecule. The structural formula tells you how many of each type of atom are in a molecule and also how they are connected. For example, here is the structural formula of ethanol.
Chemical Formula
You will also see the term chemical formula. The chemical formula tells you how many of each type of atom are in a molecule. For example, the chemical formula for ethanol is
C2H6O.
Notice that this is less information than the structural formula (but more compact). You must be careful not to confuse substances that have the same chemical formula. For example, ethanol and dimethyl ether have the same chemicial formula (i.e.C2H6O).
Lewis Dot Ionic Bonding Worksheet
Their chemical formulas are identical, but their structural formulas and their physiological effects are markedly different.
Empirical Formulas
An empirical formula (simplest formula) tells us the simplest whole number ratio of atoms in a molecule. When identifying an unknown pure substance, chemists will often start by performing experiments to determine the empirical formula of the substance.
For example, hydrogen peroxide's chemical formula is H2O2, but its empirical formula is HO.
The chemical formula for glucose is C6H12O6, but its empirical formula is CH2O, and its structural formula is
Now, let's try some sample quiz questions on Empirical Formulas:
Ionic Bonding Lewis Dot Diagram
Homework from Chemisty, The Central Science, 10th Ed.
2.39, 2.41, 2.43, 2.45, 2.53, 2.55Mechanical fuel pump pressure regulator
Lewis Dot Structure for Boron Oxide (B2O3) Boron (metal) has 3 valence electrons Oxygen (nonmetal) has 6 valence electrons, needs 2 to be balanced B3↘ ↙O2 B2 O3 Lewis Dot Structure for Sodium Fluoride Na +1 Cl-1 Na has 1 valence electron Fl needs 1 to be balanced Na Cl Covalent Bonds Covalent Bonds are attractions of 2 nonmetals They have low melting/boiling points and aren't a good ... BCl3. Back. 70 More Lewis Dot Structures. Note**Boron is an exception and will not form an octet. Contains 3 bonding pairs and no lone pairs. This correlates with the property that it is dangerously reactive. Note- BF3,BBr3,BI3 are the same shapes.
Comments are closed.